We were measuring the wrong thing too — until our children's skin forced us to ask a better question.
As a dentist and a biomedical engineer, we understood germ kill before we understood what germ kill was costing the skin doing it. We recommended handwashing to patients. We followed CDC protocols at home. We selected the gentlest verified formulas we could find for our children.
Our daughter's skin still reacted. Every time. After every wash.
What two years of formulation research revealed:
"Kills 99.9% of germs" leaves dead residue and harsh chemicals on skin after every application
The FDA removed the two most common antibacterial germ-kill agents from consumer soap in 2016 — citing safety concerns and lack of evidence they outperformed plain soap and water
Physical removal of germs is more complete than chemical killing — nothing left behind means nothing left to transfer
The wet-dry cycle required by every traditional soap — including hypoallergenic formulas — compounds barrier damage across every daily wash regardless of what the formula contains
Swiss laboratory testing using ASTM E1174 medical-grade protocols confirmed NOWATA physically removes 99.9% of bacteria and viruses — without the chemistry causing the damage
The question we could not stop asking after our daughter's first reaction: what if the mechanism we use to clean our hands is causing more damage than the germs we are trying to remove?
This page is our answer. Not the gentlest compromise we could formulate. The most effective hypoallergenic hand soap solution we could verify.
TL;DR Quick Answers
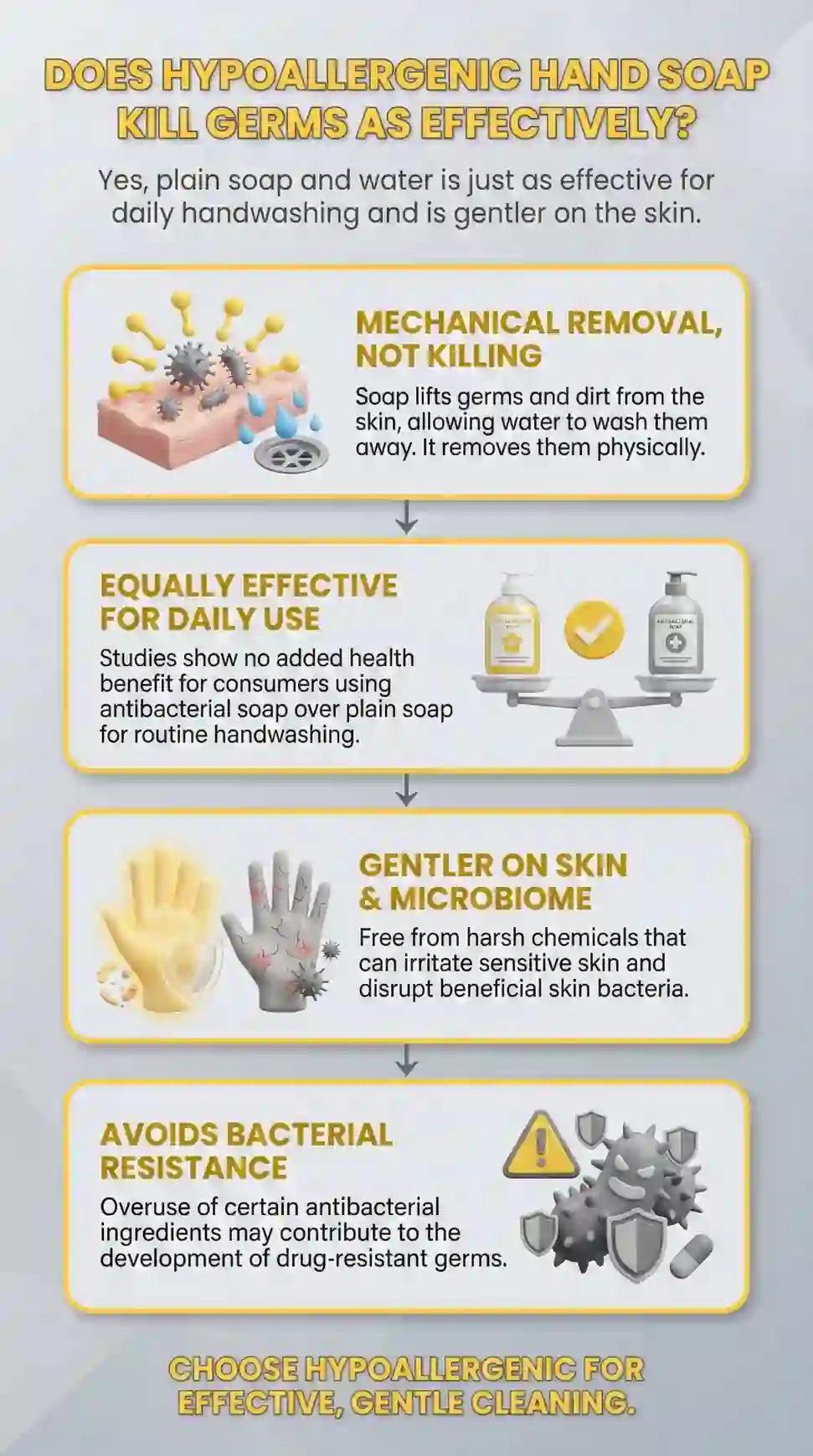
Does Hypoallergenic Hand Soap Kill Germs as Effectively as Conventional Soap?
The better question: does it remove them as completely? After two years of formulation research and independent laboratory verification, our answer is yes — and removal is the more complete standard.
What the regulatory and scientific record confirms:
FDA banned the two most common antibacterial germ-kill agents from consumer soap in 2016
Manufacturers could not prove they outperformed plain soap and water in real-world conditions
CDC's own handwashing science is built on physical removal through friction — not chemical kill rate
Kill rate was never the complete measure of effective hand hygiene
What independent Swiss laboratory testing using ASTM E1174 confirmed for NOWATA:
99.9% physical removal of bacteria including E. coli
99.9% physical removal of viruses including Murine Norovirus — a human norovirus surrogate
Complete removal — no residue, no harsh chemistry, nothing left behind on skin
Why physical removal outperforms chemical killing:
Chemical killing leaves dead germ residue and harsh chemicals on skin after every wash
That residue transfers to every surface and person touched afterward
Physical removal takes everything with it — nothing left behind means nothing left to transfer
Intact skin barrier provides primary pathogen defense between washes — chemical killing damages it, physical removal preserves it
What we learned after our daughter reacted to every formula carrying a germ-kill claim:
Kill rate and barrier damage are produced by the same mechanism simultaneously
Removing that mechanism removed both problems at once
The trade-off between gentle and effective is a false choice built on the wrong measure of clean hands
NOWATA was built on that conclusion. Verified independently before we trusted it. Tested on our own children before anyone else's.
Top Takeaways
The FDA concluded antibacterial soap was no more effective than plain soap and water — and most consumers never found out.
2016: FDA banned 19 active antibacterial ingredients from consumer hand soap
Triclosan and triclocarban — the two most widely used agents — were removed
Manufacturers could not prove safety for long-term daily use
Manufacturers could not prove they outperformed plain soap and water
We recommended antibacterial soap to patients for years — and trusted labels longer than the regulatory record warranted
Kill rate was never the complete measure of effective hand hygiene
Physical removal of germs is more complete than chemical killing — and the CDC's own science agrees.
Soap lifts germs off skin — it does not kill them
CDC handwashing guidance is built on physical removal — not chemical kill rate
Chemical killing leaves dead germ residue and harsh chemicals on skin
That residue transfers to every surface and person touched afterward
Physical removal takes everything with it — nothing left behind means nothing left to transfer
Swiss lab ASTM E1174 testing confirmed: 99.9% physical removal of bacteria and viruses
Removal is what the CDC's own science identifies as the foundation of effective hand hygiene
Barrier damage from frequent handwashing creates its own infection risk — making barrier protection a hygiene benefit, not a comfort trade-off.
NIH research: repeated handwashing creates micro-fissures in the skin surface
Micro-fissures harbor bacteria soap cannot reach during subsequent washes
Compromised barrier increases pathogen penetration between handwashing events
8 to 10 daily washes: 51% increased hand eczema risk — regardless of soap type
Every formula damaging the barrier in the name of germ protection undermines the body's own primary defense
We stopped accepting that trade-off when we understood what it was costing our daughter's skin
We built NOWATA around eliminating it entirely
The trade-off between gentle and effective is a false choice built on the wrong measure of clean hands.
Kill rate does not account for residue left on skin after killing
Kill rate does not account for barrier damage the killing chemistry causes
Kill rate does not account for what the body's own barrier contributes between washes
A hypoallergenic formula built around physical removal is not a compromise on effective hand hygiene
It is alignment with the mechanism the CDC's own science identifies as the foundation of handwashing
Gentle and effective are not opposing goals — they are the same goal achieved through a more complete mechanism
We built NOWATA to prove it — because our daughter's skin gave us no other option
Doctor-created, independently verified, and built around removal — not killing — is the standard hypoallergenic hand soap should be held to.
"Hypoallergenic" has no federal regulatory definition — any brand can use it without proof
"Kills 99.9% of germs" requires independent verification with a named protocol to mean anything clinically
NOWATA formulated by a dentist and a biomedical engineer — parents of children with reactive skin
Swiss lab ASTM E1174 verification: 99.9% physical removal of bacteria and viruses — confirmed independently
Zero SLS, alcohol, synthetic fragrances, parabens, and dyes — verified by ingredient list not front-label claim
No wet-dry cycle — barrier integrity preserved across every daily application
Tested on our own children before anyone else's
That accountability is not a marketing claim — it is the only standard we have ever trusted
Most people assume killing germs and removing them are the same thing. As healthcare professionals, we understood the distinction before we started formulating. As parents watching our daughter react to products we believed were safe, we understood why it mattered.
What chemical germ killing actually does:
Targets pathogens with active chemical agents — triclosan, alcohol, benzalkonium chloride
Denatures germ proteins on contact — the kill mechanism most labels reference
Leaves dead germ residue on skin after every application
Leaves the harsh chemicals that did the killing behind with the residue
Transfers both to every surface and person touched afterward
What that residue means for skin that needs to stay intact:
Dead germ material is not inert — it remains on the skin surface after the active ingredient dissipates
Harsh chemical agents left behind continue interacting with skin barrier lipids after washing
For reactive skin, the residue is an additional sensitizer layered on top of the damage the wash cycle already created
Killing germs and leaving them on skin is not the same as removing them
What we kept returning to during two years of formulation research:
The most common measure of soap effectiveness — kill rate — does not account for what remains after killing
A formula that removes 99.9% of germs entirely leaves nothing behind to transfer or continue irritating
Physical removal is the more complete standard — and the one we built NOWATA around
What the FDA's 2016 Ruling Revealed About Antibacterial Germ-Kill Claims
As clinicians we followed regulatory updates. As parents trusting antibacterial labels, we did not fully appreciate what the 2016 FDA ruling meant until we started formulating.
What the FDA concluded after review:
Banned 19 active antibacterial ingredients from over-the-counter consumer soap
Triclosan and triclocarban — the two most widely used antibacterial agents — were among those removed
Manufacturers could not demonstrate these ingredients were safe for long-term daily use
Manufacturers could not demonstrate they outperformed plain soap and water in real-world conditions
Documented concerns included hormone disruption and contribution to antibiotic resistance
What this revealed about germ-kill claims more broadly:
The strongest chemical germ-kill agents in consumer soap failed to prove superiority over plain soap and water
The efficacy premise behind antibacterial soap — more chemical kill equals better hand hygiene — was not supported by the evidence manufacturers were required to provide
Chemical killing is not the gold standard it has been marketed as for decades
What this meant for how we approached NOWATA's formulation:
We stopped asking which chemical killed most effectively
We started asking how to remove germs completely — without leaving anything behind
Physical removal through clumping technology became the mechanism we built around
The question shifted from kill rate to removal rate — and the answer changed everything
Why Physical Removal Outperforms Chemical Killing
This is the insight that took two years of research and one daughter's reactive skin to fully understand. It is also the one we could not find articulated anywhere on any label we read during that time.
What physical removal means in practice:
NOWATA's clumping technology binds to germs, dirt, and oil on contact
Rubbing hands together causes the formula to form small clumps trapping contaminants
Brushing clumps away removes everything — germs go with them entirely
No dead residue left on skin. No harsh chemicals left behind. Nothing to transfer.
Why removal is more complete than killing for skin that needs to stay intact:
Killing leaves dead germ material on skin — removal takes the material with it
Killing leaves chemical agents on skin — removal leaves nothing
Killing transfers residue to every surface and person touched — removal transfers nothing
A compromised skin barrier from harsh chemistry is itself a hygiene risk — micro-fissures harbor bacteria that soap cannot reach
What independent Swiss laboratory testing using ASTM E1174 medical-grade protocols confirmed:
99.9% physical removal of bacteria including E. coli
99.9% physical removal of viruses including Murine Norovirus — a human norovirus surrogate
Complete removal with no water, rinsing, towels, or residue left on skin
The same result achieved without the chemistry causing barrier damage in reactive skin
What this meant as healthcare professionals who needed to trust the result:
Gentle meant nothing to us if it did not also mean verified
We would not release a product without independent confirmation the removal claim was real
The Swiss laboratory result was not a marketing decision — it was the minimum standard we required before the formula left our hands
Why Hypoallergenic Soap Can Be Both Gentle and Effective
The assumption behind most searches for this topic: gentler formulas are less effective. We spent two years proving that assumption wrong — because our children needed us to.
Where the assumption comes from:
Traditional soap effectiveness is measured by chemical kill rate
Removing harsh chemistry from a formula appears to reduce the mechanism responsible for that kill rate
The logical conclusion: hypoallergenic equals less effective
Why that logic is built on the wrong premise:
Chemical killing is not the only — or most complete — mechanism for effective hand hygiene
The FDA concluded that antibacterial chemistry did not outperform plain soap and water
Physical removal outperforms chemical killing by eliminating residue entirely — not just denaturing pathogens and leaving them behind
A genuinely hypoallergenic formula built around physical removal does not trade effectiveness for gentleness — it achieves both through a different mechanism, and a vegan zero-waste hand soap built to that standard delivers the same clean without leaving sensitive skin paying the price.
What we built into NOWATA to prove both simultaneously:
Zero SLS, alcohol, synthetic fragrances, parabens, and dyes — genuinely hypoallergenic by ingredient not just label
Clumping technology physically removing 99.9% of bacteria and viruses — verified independently
No wet-dry cycle — eliminating the mechanical barrier damage that compounds across every daily wash
Swiss laboratory verification using ASTM E1174 medical-grade protocols — the same standard applied to healthcare hand hygiene products
The result we arrived at after two years of formulation:
Hypoallergenic does not require a compromise on effectiveness
Effectiveness does not require chemistry that damages the barrier it is meant to protect
Both are achievable simultaneously — through physical removal rather than chemical killing
That conclusion required independent verification before we trusted it — and we would not share it with other families until we did
What "Effective" Should Actually Mean for Hypoallergenic Hand Soap
After two years of research, clinical credentials, and raising children with reactive skin, here is the standard we arrived at — and why the industry measure falls short of it.
What the industry currently measures:
Kill rate — percentage of pathogens denatured by chemical agents
Log reduction — the mathematical expression of that kill rate
Neither measure accounts for residue left on skin after killing
Neither measure accounts for barrier damage caused by the chemistry doing the killing
Neither measure accounts for transfer of dead germ material and chemical residue to subsequent surfaces
What effective should actually require for hypoallergenic hand soap specifically:
Complete removal rate — not kill rate — as the primary efficacy measure
Zero residue standard — nothing left on skin after application
Barrier integrity preservation — skin left intact to continue its protective function between washes
Independent verification — third-party laboratory confirmation of removal claims using named protocols
Reactive skin testing — efficacy confirmed on the skin type the formula claims to protect
What NOWATA was built to deliver against that standard:
99.9% physical removal — verified by independent Swiss laboratory testing using ASTM E1174
Zero residue — clumping technology lifts everything off entirely
No wet-dry cycle — barrier integrity preserved across every daily application
Tested on reactive skin — our own children's before anyone else's
Independent verification — not a self-declared claim on a front label
The honest answer to whether hypoallergenic hand soap kills germs as effectively: it depends entirely on what it effectively means. If effectively means chemical kill rate — hypoallergenic formulas built around physical removal measure differently. If effectively means complete removal of pathogens with nothing left behind on skin that needs to stay intact — physical removal is the more complete standard, especially for a waterless hand soap. We built NOWATA around the second definition. Not because it was easier to achieve. Because it was the only one that answered the question our daughter's skin was asking.
"As a biomedical engineer the distinction between killing germs and removing them was always intellectually clear to me. What I did not appreciate until I was formulating for my own children was how consequential that distinction becomes for skin washing repeatedly throughout a school day. Killing leaves something behind — dead germ material and the residue from the chemistry that did the killing. For a child with reactive skin, that residue is an additional sensitizer layered on top of barrier damage the wash cycle already created. When the FDA removed the two most common antibacterial agents from consumer soap in 2016 — citing lack of evidence they outperformed plain soap and water — it confirmed what we had already observed at home. The kill rate metric the entire industry is built around was never the complete measure of effective hand hygiene. We built NOWATA around removal rate instead. Not because it tested better on paper. Because it was the answer our daughter's skin required — and the one no product on any shelf was providing."
Essential Resources
1. The Federal Ruling That Exposed What Antibacterial Germ-Kill Claims Were Actually Worth
We followed this regulatory process as clinicians and still trusted antibacterial labels longer than we should have. The FDA's 2016 final rule banned 19 antibacterial ingredients from consumer hand soap after manufacturers failed to prove they were safe for long-term daily use or more effective than plain soap and water. Before trusting any germ-kill claim on any hand soap label — read this ruling first. https://www.fda.gov/drugs/drug-safety-and-availability/fda-issues-final-rule-over-counter-antibacterial-soaps
2. The CDC Explains Why Physical Removal — Not Chemical Killing — Is the Foundation of Hand Hygiene Science
This is the resource that reframed how we think about germ removal entirely — and it came from the agency whose handwashing guidance we had been following for years without fully reading the science behind it. The CDC confirms that physical removal of germs through friction is the mechanism behind plain soap and water's effectiveness — not chemical kill agents. The federal scientific foundation for understanding why removal rate is the more complete measure of hand hygiene effectiveness. https://www.cdc.gov/clean-hands/about/index.html
3. The Research That Proves the Wash Mechanism Matters as Much as the Formula
This is the study that stopped us cold — not because the finding surprised us as clinicians but because we recognized our daughter in every data point. NIH peer-reviewed meta-analysis of tens of thousands of individuals confirmed handwashing frequency raises hand eczema risk by 51% at just 8 to 10 daily washes — regardless of soap type. The clinical foundation for understanding why eliminating the wet-dry cycle addresses the trigger no formula change alone can reach. https://pmc.ncbi.nlm.nih.gov/articles/PMC9111880/
4. Why "Hypoallergenic" on a Germ-Removing Hand Soap Means Nothing Without Independent Verification
We trusted this word for years. Our daughter's skin kept reacting. The FDA confirms "hypoallergenic" has no federal regulatory definition — any manufacturer can use it without a single clinical test for safety or efficacy. This is the document every consumer needs to read before trusting any hypoallergenic germ removal claim — gentleness and effectiveness both require independent verification, not self-declaration. https://www.fda.gov/cosmetics/cosmetics-labeling/hypoallergenic-cosmetics
5. The Clinical Science Behind Why an Intact Skin Barrier Is a Hygiene Benefit — Not Just a Comfort One
As a biomedical engineer Yalda understood this research before we started formulating. As parents watching our daughter's hands deteriorate across a school day of repeated washing, we understood why it had to become the foundation of what we built. NIH peer-reviewed research confirms that repeated handwashing causes transepidermal water loss, lipid barrier disruption, and micro-fissures that harbor bacteria soap cannot reach. Protecting the barrier is not separate from effective hand hygiene — it is part of the same goal. https://pmc.ncbi.nlm.nih.gov/articles/PMC3005527/
6. The Only Independent Verification System That Confirms Both Safety and Efficacy for Sensitive Skin
After cycling through every product claiming to be both gentle and effective — and watching our daughter react to all of them — we learned that third-party verification is the only claim worth trusting. The National Eczema Association Seal of Acceptance directory is the only resource where hand soaps are evaluated against a clinical standard for both safety and effectiveness — not self-declared by the brand selling them. When both gentleness and germ removal need to be verified independently, this directory is where informed decisions start. https://nationaleczema.org/eczema-products/
7. The Medical-Grade Testing Protocol Behind Verified Germ Removal Claims
When we developed NOWATA, independent verification using a named medical-grade protocol was non-negotiable. Gentle meant nothing to us if it did not also mean verified. ASTM E1174 is the medical-grade protocol used to measure hand hygiene product efficacy in healthcare settings — the standard behind our Swiss laboratory verification of 99.9% germ removal. Understanding what this protocol actually confirms — versus general marketing language about kill rate — is the difference between a verified claim and a self-declared one. https://pubmed.ncbi.nlm.nih.gov/15287162/
These evidence-based resources clarify why verified germ removal, barrier protection, and independent testing matter more than marketing claims—helping consumers evaluate hand soaps with the same scrutiny used when comparing top flooring options for durability and long-term performance.
Supporting Statistics
We did not arrive at physical removal as a mechanism by accident. Two years of research kept pointing to the same conclusion — the industry's measure of effectiveness was never the complete measure of effective hand hygiene. Here is the data that forced us to stop asking which soap killed germs best — and start asking what clean hands actually required.
1. The FDA Concluded Antibacterial Soap Was No More Effective Than Plain Soap and Water — After Decades of Consumer Trust
We recommended antibacterial soap to patients for years. We trusted germ-kill claims without questioning the evidence. Then we read the regulatory record as parents — not just as clinicians — and the evidence was not there.
What the FDA's final ruling confirmed:
2016: FDA banned 19 active antibacterial ingredients from over-the-counter consumer hand soap
Triclosan and triclocarban — in the majority of antibacterial consumer products — were removed
Manufacturers could not prove safety for long-term daily use
Manufacturers could not prove they outperformed plain soap and water in real-world conditions
Documented concerns: hormone disruption and antibiotic resistance at the population level
What the ruling revealed that two decades of patient counseling had not:
The strongest chemical germ-kill agents in consumer soap failed independent efficacy review
More chemical kill does not equal better hand hygiene — the evidence did not support it
Chemical killing is not the gold standard it has been marketed as for decades
The FDA confirmed it — quietly — in 2016
What changed in how we formulate after reading this:
We stopped asking which chemistry killed most effectively
We started asking how to remove germs completely — without leaving chemistry or residue behind
Physical removal became the mechanism we built NOWATA around
We verified it independently — because the regulatory record taught us labels cannot be trusted to do that
We share this not to alarm but to reframe. If the FDA concluded the category's core premise was unproven — that conclusion deserves to be part of every consumer's decision. It was not part of ours until we went looking for it ourselves.
Source: U.S. Food and Drug Administration https://www.fda.gov/drugs/drug-safety-and-availability/fda-issues-final-rule-over-counter-antibacterial-soaps
2. CDC Handwashing Science Confirms Physical Removal — Not Chemical Killing — Is the Foundation of Effective Hand Hygiene
This is the finding we had technically known as clinicians for years. What we had never done was follow it to its logical conclusion for our own children's hand hygiene.
What the CDC's handwashing science confirms:
Soap does not kill germs — it lifts them off skin
The mechanism behind plain soap and water's effectiveness is physical — friction loosens germs from skin
Rinsing removes loosened germs — physical removal is where efficacy is achieved
Proper handwashing prevents 30% of diarrhea-related illnesses and 20% of respiratory infections
The protective benefit comes from removal — not from chemical agents killing pathogens on contact
What this confirmed that our clinical training had not fully connected:
CDC hand hygiene guidance is based on physical removal science — not chemical kill rate
Adding antibacterial chemistry did not improve outcomes sufficiently to justify safety trade-offs
Physical removal is not an alternative to effective hand hygiene — it is the scientific foundation of it
What this meant for NOWATA's development:
We were not departing from hand hygiene science when we built around physical removal
We were returning to it — and eliminating the chemistry the regulatory record had already questioned
Swiss laboratory testing using ASTM E1174 confirmed: 99.9% physical removal of bacteria and viruses
Same outcome the CDC's guidance is built around — without the wet-dry cycle or barrier-damaging chemistry
The insight we could not stop returning to:
A hypoallergenic formula built around physical removal is not a compromise on effective hand hygiene
It is alignment with the mechanism the CDC's own science identifies as the foundation of handwashing
The trade-off between gentle and effective is built on a premise the federal science does not support
Source: Centers for Disease Control and Prevention https://www.cdc.gov/clean-hands/about/index.html
Source: CDC Handwashing Facts and Data https://www.cdc.gov/clean-hands/data-research/facts-stats/index.html
3. NIH Research Confirms Barrier Damage From Frequent Handwashing Creates Its Own Infection Risk — Making Barrier Protection a Hygiene Benefit
This is the research that completed the picture for us — and made the connection between hypoallergenic formulation and genuine hand hygiene effectiveness impossible to ignore.
What NIH peer-reviewed research on skin barrier function confirms:
Repeated handwashing causes measurable transepidermal water loss with every wash-and-dry cycle
Lipid barrier disruption from frequent washing creates micro-fissures in the skin surface
Micro-fissures harbor bacteria that soap — traditional or hypoallergenic — cannot reach during subsequent washes
Compromised barrier increases penetration of pathogens and allergens between handwashing events
The skin barrier is the body's primary physical defense against environmental pathogens
Compromising it through frequent washing undermines the hygiene goal handwashing is meant to achieve
What this meant when we applied it to our daughter's school day:
Six washes with barrier-damaging soap was not protecting her
It was progressively dismantling the defense system doing the actual work between washes
Infection risk created by a compromised barrier is not addressed by any chemical kill agent
Protecting her barrier was not a comfort decision — it was a hygiene decision
We had been measuring the wrong outcome the entire time
What NIH handwashing frequency research added:
8 to 10 daily washes: 51% increased hand eczema risk — regardless of soap type
Each wash-and-dry cycle compounds barrier damage before recovery can begin
Frequent handwashers absorb compounding barrier damage across dozens of daily cycles
The barrier being damaged is the same barrier providing primary pathogen defense between washes
What this confirmed for every NOWATA formulation decision:
Eliminating barrier-damaging chemistry — a hygiene decision, not a gentleness feature
Eliminating the wet-dry cycle — a clinical decision, not a convenience feature
Preserving the skin barrier between washes preserves the body's own first line of defense
99.9% physical germ removal combined with intact barrier protection is a more complete standard than chemical kill rate alone
The connection between barrier integrity and infection risk is one the hand hygiene conversation has not made clearly enough. Our daughter's skin forced us to understand it. Once we did — we could not build NOWATA any other way.
Source: National Institutes of Health — PubMed Central — Skin Barrier Function https://pmc.ncbi.nlm.nih.gov/articles/PMC3005527/
Source: National Institutes of Health — PubMed Central — Handwashing Frequency and Hand Eczema https://pmc.ncbi.nlm.nih.gov/articles/PMC9111880/
Source: National Institute of Arthritis and Musculoskeletal and Skin Diseases — Atopic Dermatitis https://www.niams.nih.gov/health-topics/atopic-dermatitis
Final Thought & Opinion
People ask us whether hypoallergenic hand soap kills germs as effectively as conventional soap. After two years of formulation research, three independent clinical credentials, Swiss laboratory verification, and raising children with reactive skin — our answer is the same every time:
You are measuring the wrong thing.
What Two Years of Research and Parenthood Actually Taught Us
We entered this question as clinicians who understood germ theory at a molecular level. We understood kill mechanisms, log reduction, and why antibacterial chemistry had been the industry standard for decades.
None of that prepared us for the regulatory record we found when we started reading it as parents — not as clinicians.
What clinical training told us:
Antibacterial chemistry kills pathogens on contact
Higher kill rate equals better hand hygiene
More chemical agents equal more protection
The industry standard was built on sound science
What the regulatory record revealed:
FDA banned the category's two most common active ingredients in 2016
Manufacturers could not prove safety for long-term daily use
Manufacturers could not prove they outperformed plain soap and water
Kill rate was never the complete measure of effective hand hygiene
CDC's own handwashing science is built on physical removal — not chemical killing
What parenthood added that no regulatory document covered:
A daughter whose skin reacted to every formula carrying a germ-kill claim
Two years of watching chemical kill rate produce chemical barrier damage simultaneously
The realization that killing germs and leaving residue on compromised skin was not clean hands
The question nobody was asking: what if the mechanism we use to clean our hands is causing more damage than the germs we are trying to remove
Our Honest Opinion on Germ Kill vs. Physical Removal
On chemical germ killing:
Kills pathogens on contact — the mechanism is real
Leaves dead germ residue on skin after every application
Leaves harsh chemistry behind with the residue
Both transfer to every surface and person touched afterward
FDA concluded the strongest antibacterial agents did not outperform plain soap and water
The mechanism produces the result — and the damage — simultaneously
On physical removal:
Lifts germs, dirt, and oil off skin entirely through clumping technology
Brush the clumps away — everything goes with them
No dead residue. No harsh chemistry left behind. Nothing to transfer.
CDC's own hand hygiene science is built on this mechanism — not chemical kill rate
Swiss lab ASTM E1174 testing confirmed: 99.9% removal of bacteria and viruses
More complete than killing — because nothing is left behind
On barrier protection as a hygiene benefit:
NIH research confirms repeated handwashing creates micro-fissures harboring bacteria soap cannot reach
Compromised barrier increases pathogen penetration between washes
Protecting the barrier is not separate from effective hand hygiene — it is part of the same goal
Every formula damaging the barrier in the name of germ protection undermines the body's own primary defense
We stopped accepting that trade-off when we understood what it was costing our daughter's skin
What the Industry Gets Wrong — and What Needs to Change
The hand hygiene industry has built its effectiveness standard around kill rate — a metric that does not account for what remains on skin after killing, what damage the killing chemistry causes, or what the body's own barrier contributes between washes.
What we believe needs to change:
Kill rate should not be the sole measure of hand hygiene effectiveness — removal rate and barrier integrity preservation should be required components of any complete efficacy standard
The FDA's 2016 ruling should be better communicated to consumers — the majority of people making hand soap purchasing decisions have never heard of it
Independent laboratory verification using named medical-grade protocols should be required before any germ removal or kill claim appears on a consumer label
Hand hygiene guidance should explicitly acknowledge barrier integrity as a hygiene benefit — not a comfort feature
The conversation about hypoallergenic soap effectiveness should be reframed around removal completeness — not kill rate — because removal is what the CDC's own science identifies as the foundation of handwashing effectiveness
What We Know From the Inside
The question is not whether hypoallergenic hand soap kills germs as effectively. The question is whether killing germs is the right measure of hand hygiene effectiveness in the first place.
What we know from the regulatory record:
FDA removed the strongest antibacterial agents from consumer soap — citing lack of evidence they outperformed plain soap and water
The evidence behind decades of antibacterial marketing was not sufficient when manufacturers were required to provide it
What we know from the clinical research:
CDC's handwashing science is built on physical removal — not chemical killing
NIH research confirms barrier damage from frequent washing creates its own infection risk
Protecting the barrier is a hygiene benefit — not a comfort trade-off
What we know from our daughter's skin:
Six washes with chemical germ-kill soap progressively dismantled the barrier doing the actual work between washes
Physical removal with nothing left behind was the more complete answer
Not because it tested better on paper — because it was the only answer addressing the full picture
One Final Thought
What makes NOWATA more effective for reactive skin is not the Swiss laboratory result — though we are proud of it. It is not the ASTM E1174 protocol — though we required it before releasing the formula.
The difference is that we asked a question the industry had stopped asking:
What does effective actually mean for skin that needs to stay intact?
The answer we arrived at:
Not the highest kill rate
Not the most aggressive chemistry
Not the formula eliminating the most pathogens while leaving the most damage behind
The formula removing 99.9% of germs completely — with nothing left on skin that needs to protect itself between every wash
We built NOWATA because that answer did not exist on any shelf we searched:
Every batch verified independently before it reaches anyone else's hands
Tested on our own children before anyone else's
That accountability is not a marketing position — it is the standard we required for our own children
The trade-off between gentle and effective is a false choice built on a metric that was never the complete measure of clean hands. We built NOWATA to prove it — because our daughter's skin gave us no other option, just as choosing the right flooring determines the integrity of the surface you rely on every day.
FAQ on Does Hypoallergenic Hand Soap Kill Germs as Effectively
Q: Does hypoallergenic hand soap actually kill germs effectively?
A: This was the question we asked before releasing a single bottle. Gentle meant nothing to us if it did not also mean verified.
The more precise question: does hypoallergenic hand soap remove germs as completely as conventional soap?
What independent Swiss laboratory testing using ASTM E1174 confirmed:
99.9% physical removal of bacteria including E. coli
99.9% physical removal of viruses including Murine Norovirus — a human norovirus surrogate
Complete removal — no water, rinsing, towels, or residue left on skin
Why removal outperforms killing for skin that needs to stay intact:
Chemical killing leaves dead germ residue and harsh chemicals on skin after every wash
That residue transfers to every surface and person touched afterward
Physical removal takes everything with it — nothing left behind means nothing left to transfer
CDC's own handwashing science is built on physical removal — not chemical kill rate
What the FDA confirmed about antibacterial kill claims:
2016: FDA banned 19 active antibacterial ingredients from consumer hand soap
Manufacturers could not prove they outperformed plain soap and water
Kill rate was never the complete measure of effective hand hygiene
The honest answer:
A genuinely hypoallergenic formula built around physical removal does not kill germs less effectively
It removes them more completely — with nothing left behind
That is the standard we built NOWATA around
Not because it was the easiest claim to make — because it was the only one we could verify independently
Q: What is the difference between killing germs and removing them — and why does it matter for hypoallergenic soap?
A: As a biomedical engineer and a dentist we understood this distinction before we started formulating. What we did not appreciate until formulating for our own children's reactive skin was how consequential that distinction becomes when the barrier is already compromised.
What chemical killing does:
Targets pathogens with active chemical agents
Leaves dead germ material on skin after the active ingredient dissipates
Leaves harsh chemistry behind with the residue
Both transfer to every surface and person touched afterward
For reactive skin — residue is an additional sensitizer layered on top of barrier damage the wash cycle already created
What physical removal does differently:
Clumping technology binds to germs, dirt, and oil on contact
Rubbing hands together forms clumps trapping contaminants
Brush clumps away — everything goes with them entirely
No dead residue. No harsh chemistry left behind. Nothing to transfer.
CDC's own handwashing science is built on this mechanism — not chemical kill rate
Why this distinction matters for hypoallergenic soap:
Physical removal eliminates the sensitizing residue chemical killing leaves behind
Preserves the skin barrier that chemical agents damage with every application
Achieves complete germ removal without the chemistry that makes conventional soap wrong for reactive skin
Gentle and effective become the same goal — achieved through a more complete mechanism
What our daughter's skin confirmed that the research explained:
Chemical kill rate and chemical barrier damage are produced by the same mechanism simultaneously
Removing that mechanism removed both problems at once
Physical removal was not the gentler alternative — it was the more complete answer
Q: Is hypoallergenic hand soap effective enough for healthcare workers and high-frequency handwashers?
A: As healthcare professionals this was the question we could not release NOWATA without answering. We watched high-frequency handwashing damage colleagues' and patients' skin for years. Gentle was not enough. Verified at a healthcare standard was the minimum.
What the data shows about high-frequency handwashers:
Healthcare workers wash hands 8 to 10 times per hour during patient care
NIH research: 8 to 10 daily washes raises hand eczema risk by 51% — regardless of soap type
Occupational hand eczema affects up to 30% of healthcare workers
Compromised skin creates micro-fissures harboring bacteria soap cannot reach
Infection risk from barrier damage not addressed by any chemical kill agent in any traditional formula
What Swiss laboratory testing using ASTM E1174 — the healthcare standard — confirmed:
99.9% physical removal of bacteria including E. coli
99.9% physical removal of viruses including Murine Norovirus
Complete removal — no water, rinsing, towels, or residue left on skin
Same protocol used to verify hand hygiene products in healthcare settings
Applied to a formula built around physical removal — not chemical killing
What this means for high-frequency handwashers:
Physical removal verified at 99.9% meets the same efficacy standard as conventional hand hygiene products
Eliminating the wet-dry cycle removes compounding barrier damage across dozens of daily washes
Intact skin is cleaner and safer than compromised skin — regardless of wash frequency
Protecting the barrier is not a comfort benefit — it is a hygiene benefit
What we built NOWATA to deliver:
99.9% verified germ removal — independently confirmed not self-declared
Zero barrier-stripping chemistry — no SLS, alcohol, synthetic fragrances, or parabens
No wet-dry cycle — mechanical barrier damage eliminated entirely
Gentle enough for reactive skin
Verified enough for healthcare professionals
We required both before we released it
Q: How do I know if a hypoallergenic hand soap's germ removal claims are verified or self-declared?
A: This is the question that took us two years to learn to ask correctly. We trusted self-declared claims on our own children's products longer than we should have.
Step 1 — Recognize what self-declared looks like:
"Kills 99.9% of germs" with no protocol cited — self-declared
"Clinically tested" with no laboratory named — self-declared
"Dermatologist tested" with no outcome specified — self-declared
"Hypoallergenic" with no third-party verification — legally meaningless
We found every one of these on products we trusted before we knew what to look for
Step 2 — Understand what independent verification actually requires:
Named third-party laboratory — not an internal brand testing facility
Specific protocol cited — ASTM E1174 or equivalent medical-grade standard
Both bacteria and virus removal confirmed — not assumed from one pathogen type
Results applicable to the formula being sold — not extrapolated from a different version
Step 3 — Cross-reference the efficacy claim against the ingredient list:
A formula claiming 99.9% removal with no clumping or binding technology deserves scrutiny
The mechanism behind the claim should be visible in the formula — not just stated on the label
If the ingredient list does not explain how the claim is achieved — the claim is not explained
Step 4 — Apply the standard we apply to our own formula:
NOWATA: independently verified by Swiss laboratory using ASTM E1174 medical-grade protocols
99.9% physical removal of bacteria and viruses — confirmed by a named third party
Zero self-declared efficacy claims — every result independently confirmed before it appears anywhere
If we would not release a claim without independent verification — no consumer should trust one without it
Q: Can hypoallergenic hand soap protect against viruses as well as bacteria?
A: Yes — and this was the verification we required before we trusted our own formula. As healthcare professionals and parents of children with reactive skin, bacterial removal alone was never sufficient. Virus removal had to be independently confirmed — or the formula was not ready.
What most consumers do not know about germ removal claims:
Many soap efficacy claims are verified against bacteria only — not viruses
Bacterial and viral removal mechanisms differ — effective against one does not mean effective against both
"Kills 99.9% of germs" without specifying bacteria and viruses separately is an incomplete claim
We found this distinction missing in the majority of product claims we reviewed during formulation research
What independent Swiss laboratory testing using ASTM E1174 confirmed for NOWATA:
99.9% physical removal of bacteria — E. coli used as the bacterial surrogate
99.9% physical removal of viruses — Murine Norovirus used as the human norovirus surrogate
Both confirmed independently by a named third-party laboratory
Complete removal — no water, rinsing, towels, or residue left on skin
Why physical removal is effective against both bacteria and viruses:
Clumping technology binds to pathogens through physical mechanisms — not pathogen-specific chemistry
Binding and removal mechanism works on both bacterial and viral particles
Brush the clumps away — bacteria and viruses go with them entirely
No pathogen-specific chemistry required — no pathogen-specific residue left behind
Why this matters for hypoallergenic formulas specifically:
Removing antibacterial and antiviral chemistry in the name of gentleness raises a legitimate question
Physical removal through clumping technology answers it independently — without the chemistry causing barrier damage
Gentle and effective against both bacteria and viruses are not opposing requirements
Independent verification using named protocols is the only way to confirm both simultaneously
What we require before trusting any germ removal claim:
Named third-party laboratory
Specific protocol for both bacteria and virus testing
Results for both pathogen types confirmed independently
Mechanism behind the claim visible in the formula
We would not release NOWATA without all four. We would not recommend any product to our own families without verifying all four. That standard is the one every family evaluating hypoallergenic hand soap deserves to apply.






